The stability of interphases has been a challenge for battery chemistry developers, who have focused on small-molecule additives to create a robust ecosystem. But, thanks to a group of researchers at the King Abdullah University of Science and Technology in Saudi-Arabia, an interesting solution could be available.
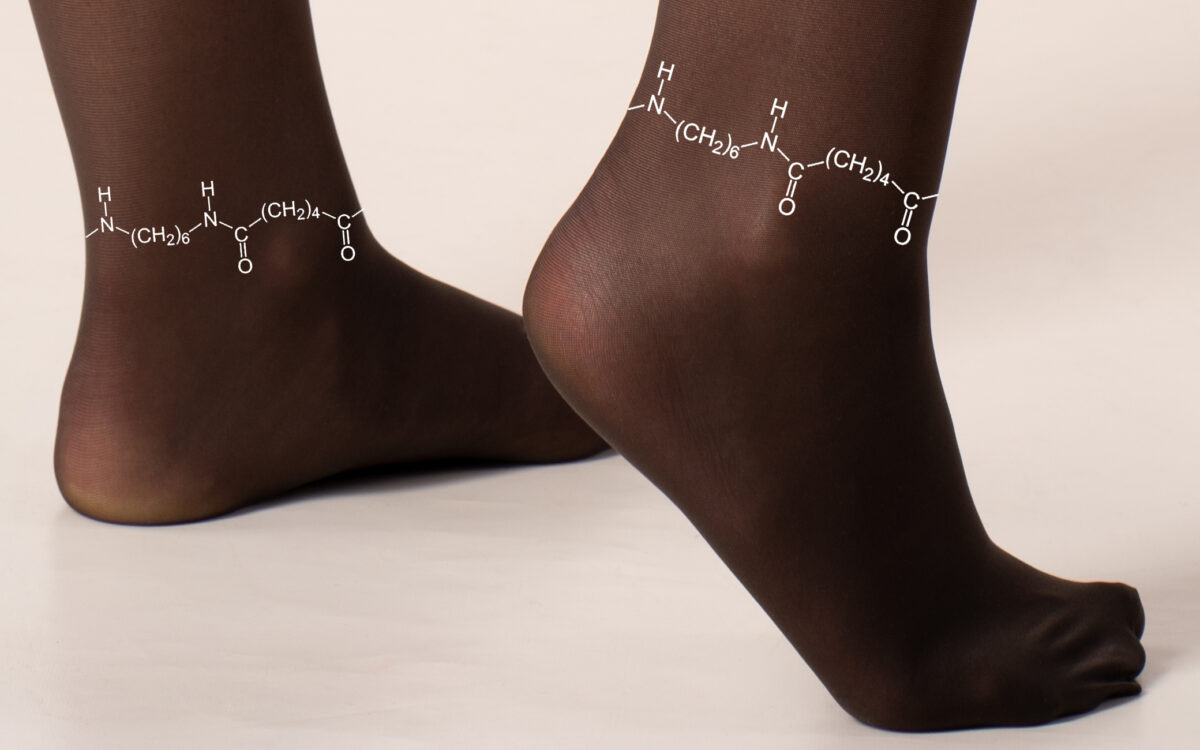
The key is using the material of pantyhose – nylon. Polyamides are macromolecules, which normally do not dissolve in conventional solvents and therefore are unsuitable for this purpose.
High-energy batteries operate under extreme potential differences exerted by their anodes and cathodes, far beyond the thermodynamic stability limits of any known electrolyte. Consequently, interphases form at the junctions between electrodes and electrolytes, providing essential kinetic stabilisation. It is well-established that even a minor amount of additives in the electrolyte can dictate robust interphase formation through targeted decomposition.
The report, published in the American Chemical Society newsletter, emphasises the need to increase the potential difference between the cathode and the anode, which leads to higher energy density. Additives used, such as unsaturated carbonate derivates, nitriles, boron, silicon and sulphur containing compounds have various disadvantages.
Macromolecular additives are attractive, offering greater thermodynamic stability than smaller molecules with analogous functional groups. The dense network of entangled polymeric chains reduces extensive contact with electrode surfaces, thereby limiting the number of available reactive sites. Possible intramolecular interactions within these chains, and with anions and solvents, may foster system stability via internal hydrogen bonds or other non-covalent forces.
An important discovery was that the macromolecule polyamide can be dissolved in mild Li+ solutions. The dissolved PA imparts a dendrite-free manner to LMA, marking a significant advancement in the development of cheaper, safer and more efficient battery additives. This research not only introduces a new chemistry for macromolecule PA dissolution but also underscores its significant potential as the macromolecule additive in augmenting the reversibility of high-energy LMBs.